The FDA advises you not to buy or use Delsam Pharma Artificial Eye Ointment.
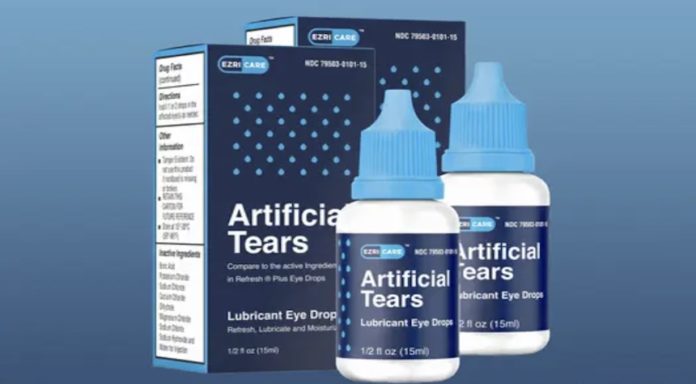
Wednesday’s announcement follows an earlier warning about EzriCare Artificial Tears and Delsam Pharma Artificial Tears regarding possible bacterial contamination. Global Pharma Healthcare is based out of Tamilnadu (India).
The FDA has criticized the company for many violations, including “lack of adequate microbial testing” and “lack of proper controls regarding tamper-evident packaging.” It also bans imports from the United States.
The FDA’s updated warning didn’t include any additional information about the over-the-counter eye ointment, other than possible bacterial contamination.
On February 1, the CDC issued an alert about an outbreak of Pseudomonas Aeruginosa (a drug-resistant strain) linked to artificial tears. EzriCare was the most popular brand of artificial tears. Five patients experienced permanent vision impairment and one died.
The CDC advises that anyone who has ever used one of these products to stop using it immediately. They should also be aware of signs of infection, such as eye pain, blurry vision, and discharge from the eyes.
Thomas Steinemann MD is the American Academy of Ophthalmology’s clinical spokesperson. He is also a professor of Ophthalmology at MetroHealth Medical Center in Cleveland. He said that while EzriCare Artificial tears and Delsam Pharma Artificial tears should be thrown out, eye drops can be safely used. There is always more to be done.
Before using eye drops, always wash your hands first, Steinemann advises. When removing the cap from the bottle, avoid touching the dropper tip to prevent transferring any bacteria from your hand. Also, do not touch the bottle to your eyes, face or nose. When finished, recap the dropper tip. If you use eye drops regularly, pay close attention to product expiration dates. “Once you open an eyedrop [bottle], you want to use it up generally in a month or so,” Steinemann says.